Describe How the Atoms in a Compound Are Held Together
Electrostatic attraction between positive and negative charges. Either ionic bonds formed when one or more electrons are transferred from one atom to another or covalent bonds formed when atoms share electrons instead of transferring them.

Ionic Bonding A Love Story L A Cartoon Guide To Formation Of Ionic Bond L Science L Brar Scribbles Ionic Bonding Chemistry Classroom Chemistry Activities
Atoms are held together in molecules by _____.

. The atoms in chemical compounds are held together by attractive electrostatic interactions known as chemical bonds. Ionic bonding As an example of another type of bond lets consider a sodium atom and a chlorine atom. The only exceptions are atoms that do not react such as Helium Neon and Argon.
Covalent compounds do not conduct electricity. Groups of covalently bonded atoms are also held together by electrostatic interactions but since the covalent bonds are so much stronger a molecular compound can exist on its own as a single molecule. In an ionic compound the cations and anions are arranged in space to form an extended three-dimensional array that maximizes the number of attractive electrostatic interactions and minimizes the number of repulsive electrostatic interactions.
The concept of chemical formulas was created to describe many characteristics of molecular compounds in a simple manner. Covalent bonding can produce much larger molecules as well. Up to 24 cash back compounds are held together by the attractive electrostatic interactions between cations and anions.
Both the bonds are considered very strong bonds. Compounds contain two or more atoms and are held together by ionic bonds whereas molecules are held together by covalent bonds. The atoms in a compound are held together by a chemical bond.
Atoms that share pairs of electrons form molecules. AAn _____ indicates the ratio of atoms in a molecule. To achieve a stable electron arrangement atoms can lose gain or share electrons.
The bond result whenever unequal sharing of electrons is called non polar. Chemical bonds are forces that hold atoms together to make compounds or molecules. The chemical bonds can be either covalent bonds or ionic bonds.
An example of a covalent compound is ammonia. Covalent bonds are strong bonds. The ions are held together in a regular spatial arrangement by electrostatic forces.
Atoms can be held together by chemical bonds. How atoms are held in place in a covalent. But there are other ways to hold atoms together.
1 In chemistry a compound is a substance that results from a combination of two or more different chemical element s in such a way that the atom s of the different elements are held together by chemical bonds that are difficult to break. DNA molecules consist of huge numbers of atoms held together mainly by covalent bonds. Molecular compounds are composed of atoms that are held together by covalent bonds.
And again the closer together they are the stronger the repulsion. A molecule is a group of atoms held together by covalent bonds. When two atoms of the same element combine to form a molecule this is called.
Basically all atoms in any compound are held together by chemical bonds. The chemical formula of ammonia is NH which tells us that in a single molecule of ammonia there is one nitrogen atom and three hydrogen atoms. Most covalent compounds consist of molecules groups of atoms in which one or more pairs of electrons are shared by at.
Ionic bonds hold atoms together using the electrostatic charge between their positive and negative ions. _____ are similar to molecular formulas but show how atoms are bonded together. Compounds can be covalent or ionic.
These bonds form as a result of the sharing or exchange of electron s among the atoms. Chemical bonds include covalent polar covalent and ionic bonds. The forces that hold atoms together are the electrical force and the strong force which is stronger than the electrical force.
The electrical force does the majority of the work of holding atoms together but the strong force helps hold in the electrical force and can somewhat override it. What force holds together an ionic compound. Collectively the forces that hold collections of molecules together are called van der Waals forces if they dont involve ions.
In covalent compounds atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. These bonds are formed when electrons are shared between two atoms. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds.
Ionic bond-1 or more electrons are transferred from 1 atom to another covalent bond- atoms share electrons moving electrons travel about the nuclei. These bonds are mainly formed by sharing of electrons or in the case when one of the elements making the compound donates electron to the other element. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero.
These ions are formed when electrons are transferred between atoms the net loss or gain determining if the ion is positive called a cation or negative an anion. A Chemical Bond is a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms togetherEnergy is the energy required to break a chemical bond and form neutral isolated atomsLikewise a Shared Pair is a pair of electrons that is shared between two atoms. Two or more elements bonded together through ionic attraction.
The closer they are together the stronger this attraction will be. _____ are compounds with the same chemical formula but different molecular structures. Covalent bonds hold atoms together because the attraction between the positively charged nuclei and the negatively charged shared electrons is greater than the repulsions between the nuclei themselves.
When elements combine through ionic bonding they will. In any atom or molecule. Two protons or two electrons will repel each other.
When atoms form bonds they can achieve a stable electron arrangement. The force responsible for combine atoms together in a compound is chemical bonding. The electrical charge that is exhibited from protons and electrons attracting to each.
The atoms in chemical compounds are held together by attractive electrostatic interactions known as chemical bonds. Describe how the atoms in a compound are held together they can be held together by an ionic and covalent bond. The atoms within a compound also must be different from eachother whereas a molecule can consist of only one element.
The atoms in a compound are held together by chemical bonds. Atoms with large differences in electronegativity transfer electrons to form ions. Now the nucleus of an atom is positively charged while electrons are negatively charged.
That is a proton and an electron will attract each other. Jon FeingershBlend ImagesGetty Images. As two atoms approach each other the electrons in their outer shells start to notice the nucleus of the other atom.

Ionic Compounds And Metals Ppt Download

Nomenclature Ionic Compounds Held Together By Ionic Bonds What Are Ionic Bonds Between Metals And Non Metals Transfer Of Electrons Between Atoms Ppt Download
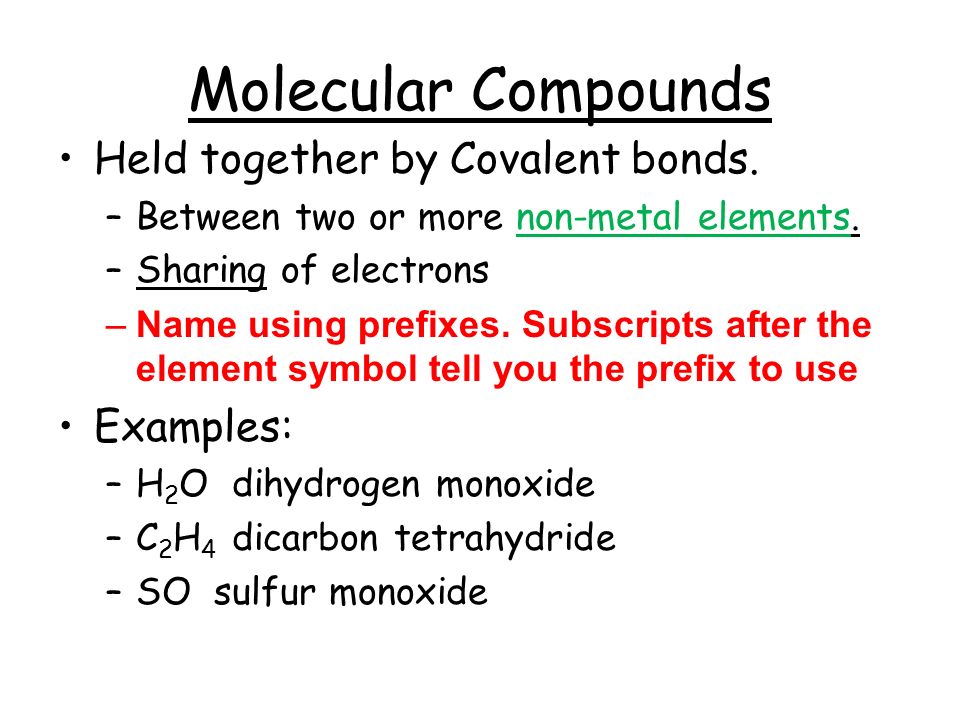
Molecular Compounds Held Together By Covalent Bonds Between Two Or More Non Metal Elements Sharing Of Electrons Name Using Prefixes Subscripts After Ppt Download

Covalent Bonding Lesson 1 Covalent Bonding Covalent Bonds Atoms Held Together By Sharing Electrons Mostly Formed Between Nonmetals Molecules Neutral Ppt Download
0 Response to "Describe How the Atoms in a Compound Are Held Together"
Post a Comment